Quiescin-sulfhydryl Oxidase-like
| Contact: | Norma Houston |
| Organization: | North Carolina State University |
| Website: | http://www.ncsu.edu/ |
| Source: | Norma L. Houston, Chuanzhu Fan, Qiu-Yun Xiang, Jan-Michael Schulze, Rudolf Jung, and Rebecca S. Boston (2005) Phylogenetic Analyses Identify 10 Classes of the Protein Disulfide Isomerase Family in Plants, Including Single-Domain Protein Disulfide Isomerase-Related Proteins. Plant Physiology 137: 762-778 |
| Criteria: | Thioredoxin domains from PDIL amino acid alignments formed a distinct, well-supported clade within the thioredoxin gene family. The QSOXL subfamily designation was given to members based on amino acid sequence homology to proteins with quiescin-sulfhydryl oxidase activity in other species (Thorpe et al., 2002). Members of the QSOXL subfamily, in addition to a TRX domain, possess an Erv1-like domain that has been independently implicated in cellular redox processes (Lange et al., 2001). |
| Gene Name: | OsQSOXL1 |
| Gene Description: | Oryza sativa quiescin-sulfhydryl oxidase-like (OsQSOXL1) |
| MSU Annotation: | OsQSOXL1 quiescin-sulfhydryl oxidase-like OsQSOXL1, expressed |
| GenBank Protein Acc: | AAT85195 |
| Comment: | AK121660 Predicted Localization1: S' Domain Composition2: thioredoxin Domain Composition2:Evr1_Alr |
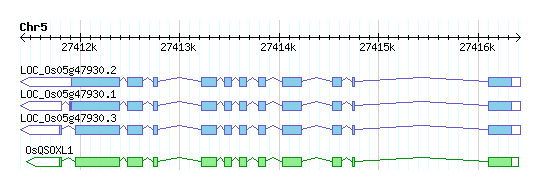
| Structural Annotation: | |
|
| |
|
1 S, Secretory pathway assignment by TargetP; (Emanuelsson et al., 2000) reliability value 0.6; S', secretory pathway assignment reliability value <0.6; O, other localization predicted. 2 Domains predicted by CDD (Conserved Domain Database) followed by number of each domain References: |
|

|

|

|
This work is supported by grants (DBI-0321538/DBI-0834043) from the National Science Foundation and funds from the Georgia Research Alliance, Georgia Seed Development, and University of Georgia.